Digital biomarkers and sensors in clinical trials are often viewed as burdensome or difficult for patients to use. But recent findings from the Actiliège NEXT study, presented at MDA 2025, tell a different story when it comes to measuring ambulatory function in young Duchenne muscular dystrophy (DMD) patients.
When comparing traditional clinical assessments (like the 6-minute walk test or NSAA) with digital health technologies (DHTs) such as the EMA qualified SV95C and stride-level data from wearable sensors, the evidence is clear: measuring movement in daily life is often more practical.
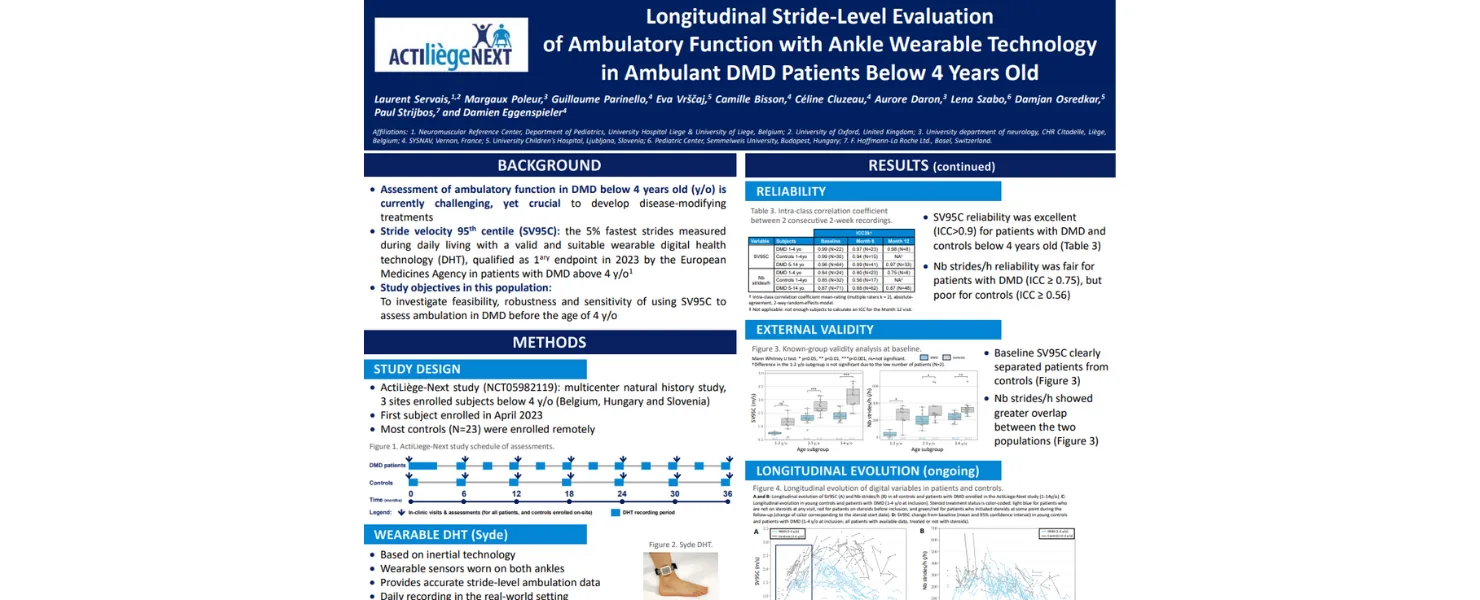
What Does the MDA 2025 Data Say?
The Actiliège NEXT study investigated ambulatory function in young patients with Duchenne muscular dystrophy (DMD) under 4 years old. This study compared traditional in-clinic assessments such as the North Star Ambulatory Assessment (NSAA), 6-minute walking test (6MWT), and stair climbing tests to physical activity endpoints captured by wearable sensors.
Key Findings
- High adherence to wearable sensors: Most patients (24 out of 26) patients in the study consistently wore ankle-worn sensors, allowing researchers to gather detailed, stride-level digital biomarkers.
- Challenges with traditional assessments: Only a subset of patients (11 to 17 out of 26) were able to fully understand or comply with in-clinic test instructions, highlighting limitations in traditional methods.
- Reliable data from wearables: The strong adherence to sensor use allowed for detailed digital variables like SV95C and stride counts per hour to be derived, offering valuable insights in an age group where traditional testing can be difficult.
Why This Matters for DMD Clinical Trials
The burden myth around wearable sensors doesn’t hold up against the data. In fact, digital health technologies may provide a more feasible way to track how patients feel and function, particularly in pediatric neuromuscular trials.
For conditions like Duchenne muscular dystrophy, especially in young children, digital health technologies offer a scalable, patient-friendly solution for activity monitoring. Rather than relying solely on clinic visits and structured tests, researchers and clinicians can now collect high-quality, real-life data without overburdening patients or families.
To learn more, download the MDA 2025 poster here!