
The Phantom Steps of ALS Patients: Why Analytical Validation Matters
During a study conducted in a controlled environment, we recorded the movements of patients with late-stage




We provide the Syde® platform, a solution for motor function assessment in clinical studies, powered by:
Pharmaceutical
companies
Patient
associations
Researchers
and physicians









Sysnav Healthcare combines regulatory expertise, precision technology, operational excellence, and scientific rigor to deliver real-world digital endpoints that accurately capture patients’ functional changes.

• We developed SV95C (Stride Velocity 95th Centile, top ambulation speed), the first digital endpoint qualified by EMA¹.
• We have a strong track record of contributions to EMA and FDA-approved clinical trial protocols.
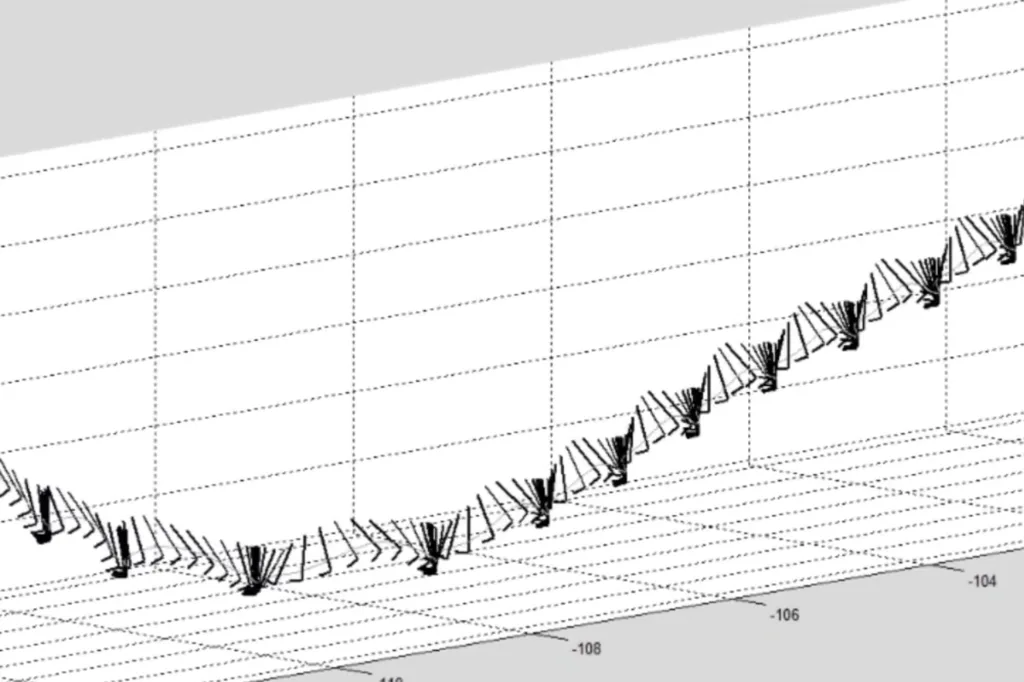
• Our DHT Syde® generates 3D reconstructions of patient movements with centimetric precision²’³ to deliver precise patient function outcomes.

• Our all-in-one solution seamlessly integrate with trial operations across all trial phases.
• We provide regulatory support, helping trials meet FDA and other authority requirements efficiently.

• We systematically assess the analytical and clinical validity of our endpoints⁴.
• Our strength lies in a deep bench of scientific and technical experts, trained at leading institutions, including Cambridge, MIT, Stanford, Ecole Polytechnique.
Schedule a meeting with our team to learn more.


During a study conducted in a controlled environment, we recorded the movements of patients with late-stage

Sysnav Healthcare was delighted to participate in the 30th Annual International Congress of the World Muscle

Last week, Sysnav Healthcare participated in the 2025 Annual NEALS Meeting in Florida. In collaboration with
By clicking on submit, you consent to being contacted by Sysnav about its products and services and you agree to the terms and conditions stated in the privacy policy.