
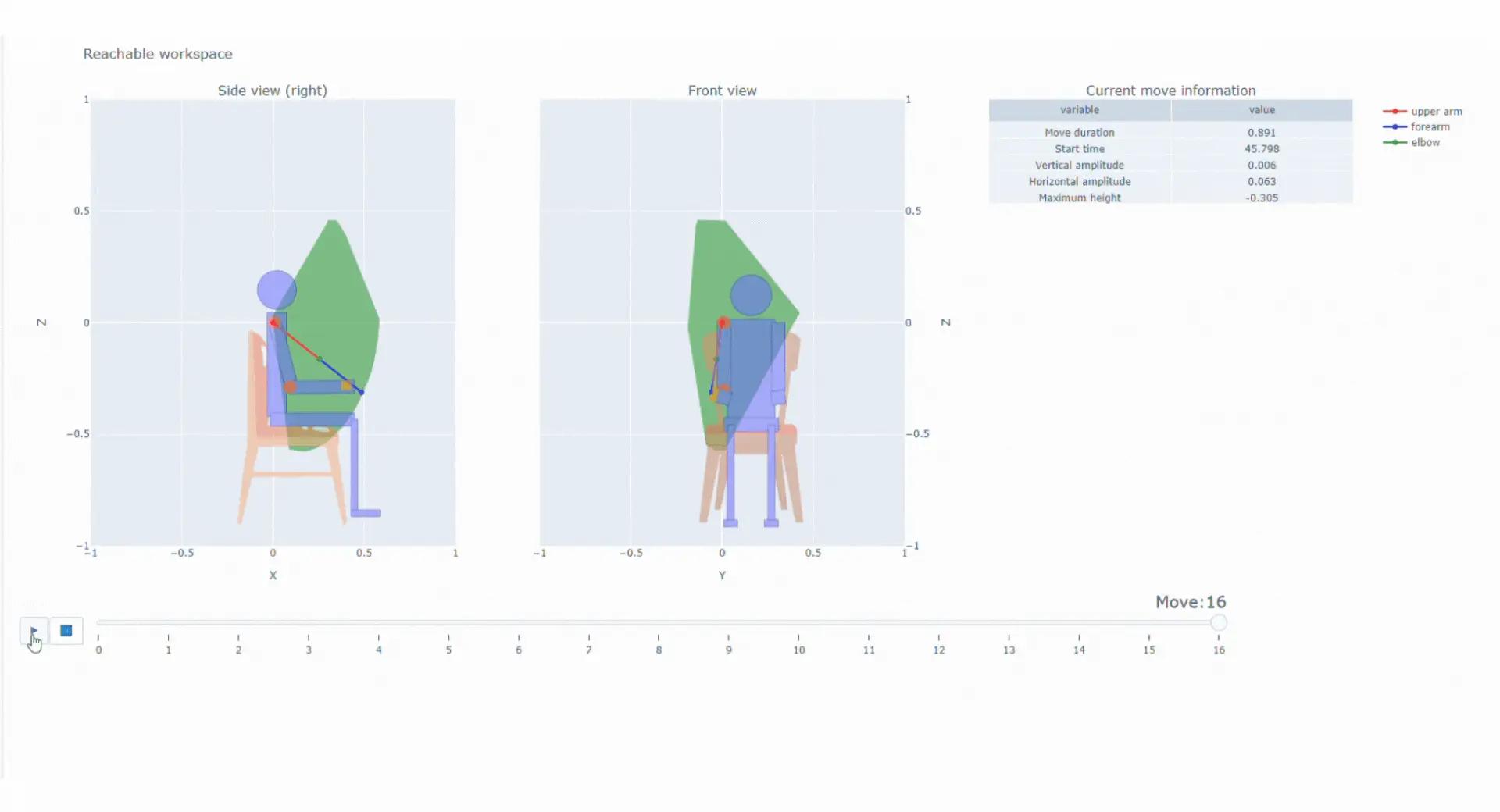
Movement is a critical aspect of neuromuscular and neurological disorders. Its precise assessment is a crucial aspect to better understand disease progression or therapeutic efficacy. Syde is a digital endpoint platform that :
Continuously captures real-world movement data
Provides meaningful variables including SV95C
Can be deployed in clinical trials
The Syde platform includes two wearable sensors for precise movement capture, a back-end IT platform with algorithms for digital endpoint calculation, and services for clinical operations.
With the data collected thanks to the magneto inertial technology and different sensors, Syde provides high precision continuous real-life data collection to reconstruct 3D trajectory and offer a wide range of possibilities regarding clinical outcome assessments
Each Syde case contains ready-to-use elements to quickly start data collection:

SYSNAV Healthcare developed a unique sensor technology to measure motion patterns, along with algorithms to transform real-world data into variables and endpoints. They represent patients’ comprehensive activity for upper and/or lower limbs in the context of clinical research. Future use might include support for diagnosis, patient stratification, therapeutic efficacy monitoring, and medical indication claims.
SV95C:
The first regulatory-qualified
real-world digital
outcome
For therapeutic, confirmatory, and natural history studies.
First regulatory-qualified real-world digital outcome measure for use as a primary endpoint in pivotal studies of Duchenne Muscular Dystrophy (DMD)
Top 5% fastest strides a patient spontaneously takes in their normal daily environment over a pre-defined time period

Sysnav Healthcare provides an all-in-one solution to assess the efficacy of a treatment complying with regulatory requirements. Syde is a complete and easy-to-use digital endpoint platform equipped with dedicated services and teams to efficiently deploy the technology, whatever the patient geographic situation and even if the HCP teams are not tech-savvy.We provide a ready-to-use report, including real-world data and a set of variables according to the study needs.
Study design
regulatory filling
according to the protocol of your clinical test
Starting of the trials
for caregivers, physicians, & medical teams to use Syde
During the trials
to clinical centers
Closure activities
for your clinical trial success


Tests for clinical trials should not be a burden on patients, their families, or clinical teams. But patient motor function is complex, with short-term variability such as fatigue and long-term evolutions such as disease progression or the effects of treatment.
Syde enables precise, continuous evaluation of motor function in real life, limiting or eliminating shortcomings and bias associated with assessments done in clinics.
Syde has been designed and improved in partnership with healthcare professionals and patient associations in order to engineer features that meet their needs and to make wearing the sensors comfortable, whatever the patient’s lifestyle, which is crucial for reliable data.
only has sensors to track mobility and nothing else, to enable our promise of privacy .
No location tracking, so no one knows where you are. No microphone, so your conversations stay private.

is at the center of the development of our devices and processes.
Syde keeps patients'
data secure and not alterable. There is no possibility of tampering or modification of data.

fully compatible, with patients' lifestyles.
Designed so that technology fit the patient
not the other way around.

are small enough to remain invisible under trousers, discrete at wrists, light with its bracelet, designed for comfort, Syde likes to go unnoticed.

are waterproof to comply with the patient's lifestyle. Syde is robust and can
withstand the shocks and bumps of everyday life.
Patients don’t have to restrain their movement
to protect the sensors.

is suitable indoor and outdoor use . It can record and store data all week long even
without an internet connection.
Data is automatically transferred and analyzed when the patient
connects the sensors to the docking station.

adapts to the patient's tastes whatever their age.
There are different options available for
Syde bracelets.