Sysnav Healthcare is grateful to see Roche presenting a poster on our Syde-derived SV95C at the 21st International Conference on Duchenne and Becker Muscular Dystrophy, hosted by Parent Project APS on 16th February 2024 in Italy.
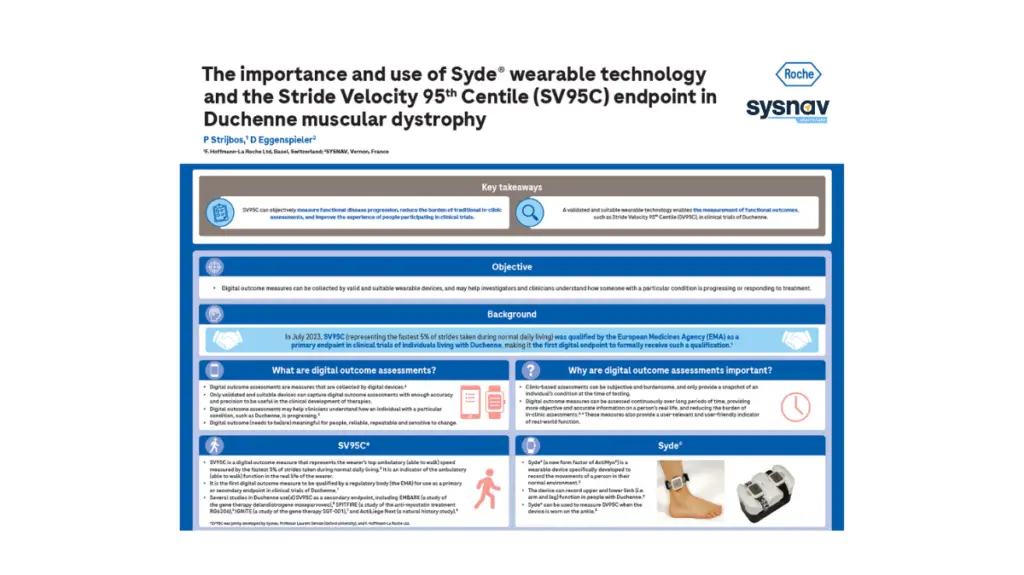
Title of the Poster: The Importance and use of Syde® wearable Technology and the Stride Velocity 95th Centile (SV95C) Endpoint in Duchenne muscular dystrophy
SV95C is the first digital endpoint to be qualified as a primary endpoint in clinical trials for Duchenne Muscular Dystrophy by a regulatory body (European Medicines Agency).
The poster highlights the importance of digital outcome measures and how clinicians and investigators can use them to precisely measure the response of patients to a treatment.
This aligns with Sysnav Healthcare’s mission to use meaningful real-world data to accelerate medical progress.
Do you want to learn more about SV95C?